Eion Explainer: Why we know that rock weathering works
Or: How the Ocean Got its Salt
I had a call with someone last year in which they said one super-major ag company wasn’t interested in Enhanced Rock Weathering because “I heard it doesn’t work”. It was one of those head-scratchers for me, like “what does that even mean, ‘doesn’t work’?” And to be sure, I heard on multiple occasions last year some variant of “too good to be true”, “foo foo dust”, or even “I have a skepticism in rocks that purport to have magic properties”. Like, if this is so great, why hasn’t anybody done it?
I want to capture here, in a brief and intuitive way, why we, “the scientific community,” know CO₂ removal by enhanced rock weathering is a real thing. Putting on my Peter Falk hat, à la Princess Bride, this story is better than you think: stay with me.
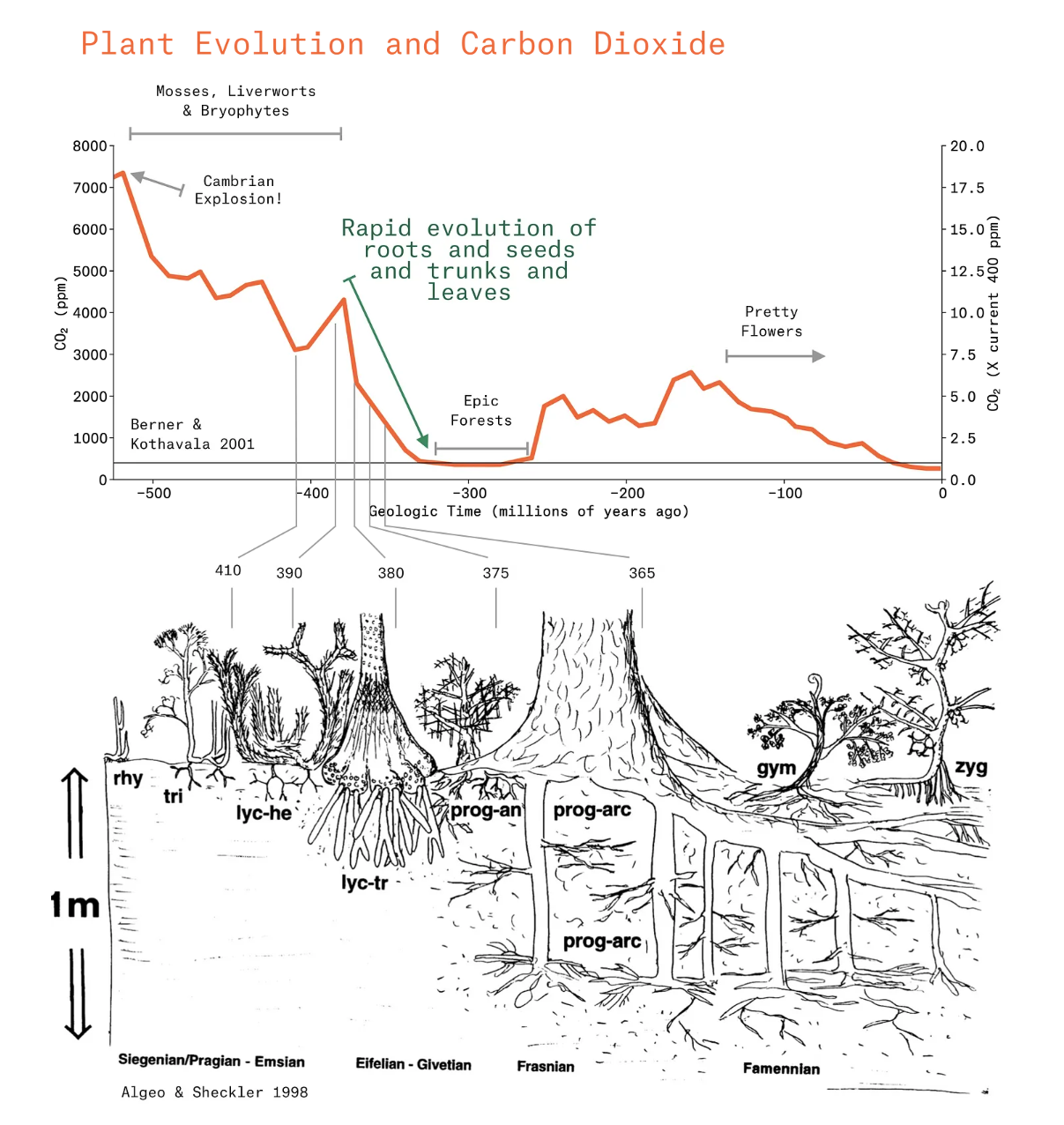
First of all, we know we have CO₂ in the air, and it’s about 420 parts per million. It turns out, that as high as that is for the last many million years, it seems that back 500+ million years ago it was way higher. Like 15–20x higher, close to 8000 parts per million. Clever people have come up with ways to determine what the CO₂ was over that long history, and we have a time series like the red curve in the figure below (from Royer et al 2004). Unpacking that curve, we can see an early period where the CO₂ is north of 4000 ppm, then a precipitous drop shortly after 400 million years ago, and it actually lands at a level of ~350ppm, just about what we have now. It swings up and down for a couple hundred million years, and then settles into its present day value (until recently about 270ppm). If you use your imagination, this curve has sharks that swallow buildings, giant dragonflies, ruthless dinosaurs, nearly all of life on Earth.
Now, the striking thing about this 👆 curve is the steep drop starting 380 million years ago. What’s going on there? First, the world is blazing hot, and then quick as you can blink, in 50 million years it’s covered in glaciers. Such is the power of rock weathering my friend. In fact, the history of this curve, which comes from Bob Berner’s landmark GEOCARB3 model, is that he couldn’t manage to make the CO₂ curve match the proxy record with physical processes alone: he needed plant evolution and rock weathering to reproduce that precipitous decline.
It’s helpful here to dust off the biology textbook (I am doing it too) and remember about algae’s evolution into mosses, bryophytes, hornworts, liverworts, just about every kind of wort, all began showing up around 500 million years ago. Steven Jay Gould gushed about that evolutionary moment in the ocean as “Wonderful Life”, but up on land it was objectively boring for about another 100 million years. Arguably, it was because the plants didn’t have to try very hard to make a living: there was so much CO₂ in the atmosphere that they didn’t need leaves or roots or stems or any of those features that reflect plant life under scarcity.
Then, around 400 million years ago plant life got competitive: plants invented roots, which helped them gather nutrients, and anchor their trunks as they started growing taller. They evolved leaves because they needed to reduce water loss as they left the pond shore. They blocked the light from their neighbors, which drove ever higher growth of their trunks. In 30 million years, plants grew from 10 inches tall to 100 feet tall. And because the forest grew so crowded, plants evolved seeds to disperse into new lands, and colonized the continents. By 300 million years ago we were at full Carboniferous, when vegetation rioted on the earth and the big trees were kings¹.
There’s a lot more evolution after that, but let’s pause there to collect our insights.
To recap: we have a rapid removal of CO₂ from the atmosphere, accompanied by a rapid expansion of plants across land. Or: rapid expansion of plants across land, accompanied by a rapid drop in CO₂. Where does rock weathering fit in? Right in the middle. All those leafy plants spread out across the land were photosynthesizing massively, sucking up CO₂ from the air. Just as today, they respire half of it out. And half of what they respire comes out the roots into the soil. That massive dose of CO₂ into the soil increased its acidity suddenly and mightily, like 1000-fold, weathering rocks that had been all but undisturbed for epochs. Those rocks were full of calcium, magnesium, potassium, and sodium, which all became dissolved into solution, and were carried by rivers into the ocean. In fact, there weren’t even rivers to speak of before this time, because the continental interiors were so dry. By recycling precipitation back into evaporation, plants brought the water inland, flushing ever more of these elements from land to sea.
The alkalinity (the calcium and magnesium etc, as measured by their charge) that entered the soil solution captured a fraction of the CO₂ that plants had respired and which was dissolved into solution. Not all of it, but just enough to balance the positive charge of the cations by the negative charge of dissolved bicarbonate. So the dissolving of the rocks and the flushing of the rivers to the oceans resulted in a massive transfer of carbon from the air to the sea. In fact, about a quarter of all the carbon in the ocean, as well as a quarter of the alkalinity of the sea, can be accounted for in this mass carbon drawdown event².
Simply put, once plants became trees, rocks started dissolving, and alkalinity flowed to the ocean, taking 90% of the CO₂ in the atmosphere with it. And that’s how you know rock weathering works. It’s why things are the way they are.